Children as young as six will be given the Oxford/AstraZeneca vaccine as part of a new clinical trial to test its efficacy in young people.
300 volunteers will take part in the research in the UK to assess whether the coronavirus vaccine will produce a strong immune response in children aged between six and 17.
The trial will begin this month at Oxford University and its partner sites in London, Southampton and Bristol.
The AstraZeneca jab is one of three to have been approved for use in adults in both Ireland and the UK, along with those from Pfizer/BioNTech and Moderna.
Professor Andrew Pollard, chief investigator on the trial, said: "While most children are relatively unaffected by coronavirus and are unlikely to become unwell with the infection, it is important to establish the safety and immune response to the vaccine in children and young people as some children may benefit from vaccination.
"These new trials will extend our understanding of control of SARS-CoV2 to younger age groups."
Up to 240 children will receive the vaccine and the others will get a control meningitis jab.
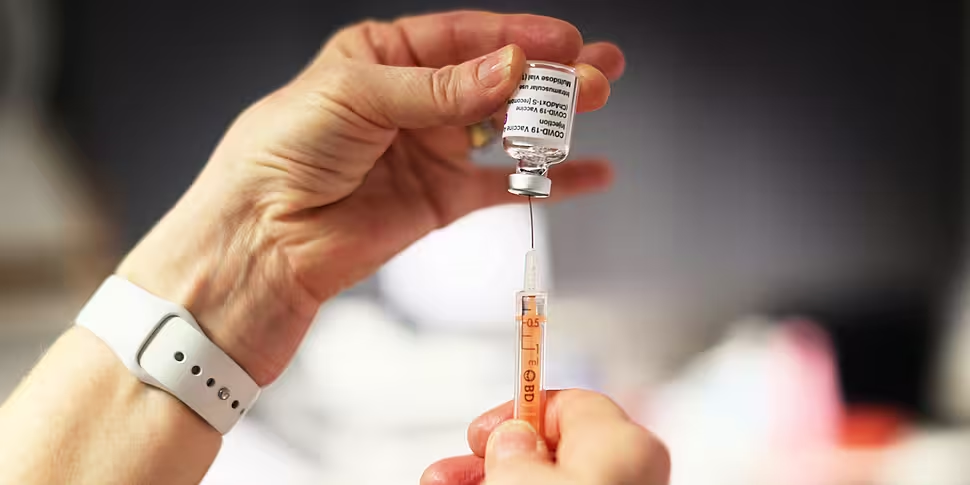
 A nurse prepares a dose of the AstraZeneca COVID-19 vaccine. Credit Olivier Chassignole, Pool via AP
A nurse prepares a dose of the AstraZeneca COVID-19 vaccine. Credit Olivier Chassignole, Pool via APThe trial is the first to assess the vaccine's efficacy in children aged six to 17.
Other research had begun but they are measuring efficacy in those aged 16 and 17, the University of Oxford said.
It comes as 248,284 doses of the vaccine have been administered in Ireland as of Tuesday.
158, 904 people have received their first dose and 89,380 people are now fully vaccinated.
The rollout of COVID-19 inoculations to over 85-year-olds will commence from Monday.
Additional reporting by IRN