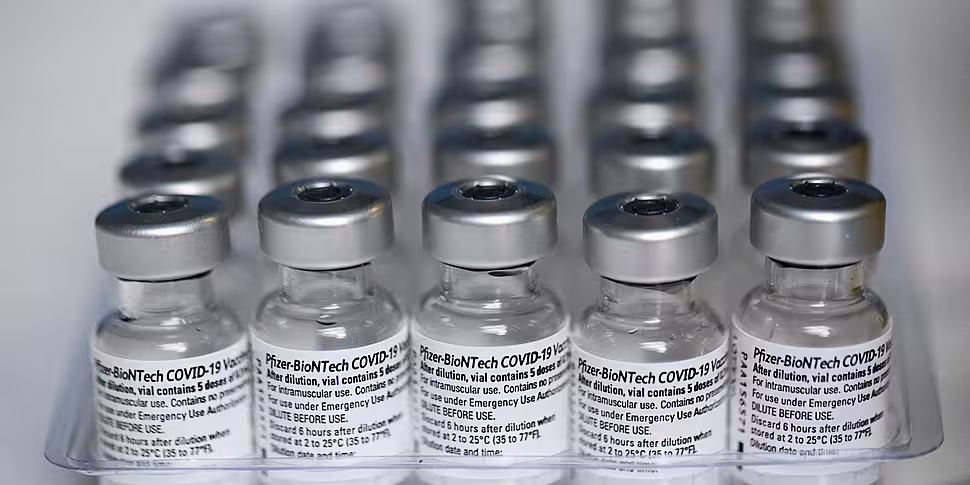
Pfizer and BioNTech say trials have shown their coronavirus vaccine to be extremely effective in teenagers aged 12-15.
The companies say a major trial has shown "100% efficacy and robust antibody responses" in the age group.
They're now hoping teenagers can start being vaccinated before the start of the next school year, and will ask the EMA to approve the use of the vaccine here 'as soon as possible'.
A phase three trial of 2,260 teenagers in the US found that the results "exceeded those recorded earlier in vaccinated participants aged 16 to 25 years old".
The company says the shot was well tolerated and side-effects were generally the same as those seen in adults.
Trials are ongoing to test the vaccine among younger children.
In a statement, Pfizer CEO Albert Bourla said: "The initial results we have seen in the adolescent studies suggest that children are particularly well protected by vaccination, which is very encouraging given the trends we have seen in recent weeks regarding the spread of the B.1.1.7 UK variant.
“We plan to submit these data to FDA as a proposed amendment to our Emergency Use Authorization in the coming weeks and to other regulators around the world, with the hope of starting to vaccinate this age group before the start of the next school year.”
The Pfizer / BioNTech jab remains the most widely used in Ireland, with over 600,000 doses administered so far.
To date, the EU and many other countries have only approved vaccines for use in adults.