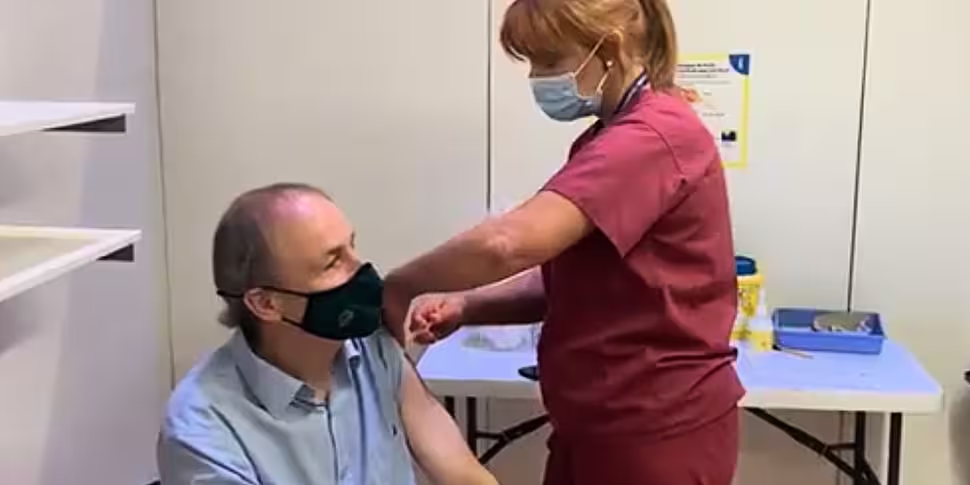
The Taoiseach has said he is "delighted" after he received his first dose of a COVID-19 vaccine today.
Micheál Martin was administered the AstraZeneca jab at the Cork City Hall vaccination centre this afternoon.
He praised the "fantastic" HSE staff and volunteers at the centre, and added that vaccines "are making a major difference as we protect the most vulnerable and open up society".
It comes after a record day on Friday when more than 52,000 doses were administered across the country.
As of Thursday, almost 1.3 million people in Ireland had received one dose of the coronavirus inoculation.
Over 25% of the EU population has now been given a first dose of the jab, with the bloc set to receive an extra 1.8 billion doses of the Pfizer vaccine between 2021 and 2023.
Delighted to get my #CovidVaccine today from the fantastic @HSELive staff and volunteers at Cork City Hall
The vaccines are making a major difference, as we protect the most vulnerable and open up society! pic.twitter.com/30EccijGY4— Micheál Martin (@MichealMartinTD) May 9, 2021
Meanwhile, a Monaghan GP has expressed concern that the message being communicated about vaccines continues to be "mixed".
Currently, there are age limits on the AstraZeneca and Johnson & Johnson vaccines due to rare blood clotting events.
Dr Illona Duffy told On the Record with Gavan Reilly: "I have concerns that the message that we're continuing to give across about vaccines is very mixed, and that's the message that's coming from NIAC, the HSE and the government.
"We know that the data out there is that all of these vaccines work and have been shown to protect," she said.
"We know that AstraZeneca and Johnson & Johnson are associated with small numbers of this unusual type of clot.
"If we look at AstraZeneca, the risk of getting one of those clots is 0.0005%, so it's tiny."
She continued: "We know with Johnson & Johnson, the single-dose vaccine which has been used extensively in the UK that of these 800 million doses there were only eight of these clots.
"In the States, they did a review of these cases and for a period of time, suspended the use of the vaccine, but from the end of April, this has been opened up again and is licensed for all of those over the age of 18.
"I think when we're saying to people, 'We may or may not give it, or we may give this vaccine if there's no other one available, it doesn't really encourage people to have faith in the vaccine in something that we know works."