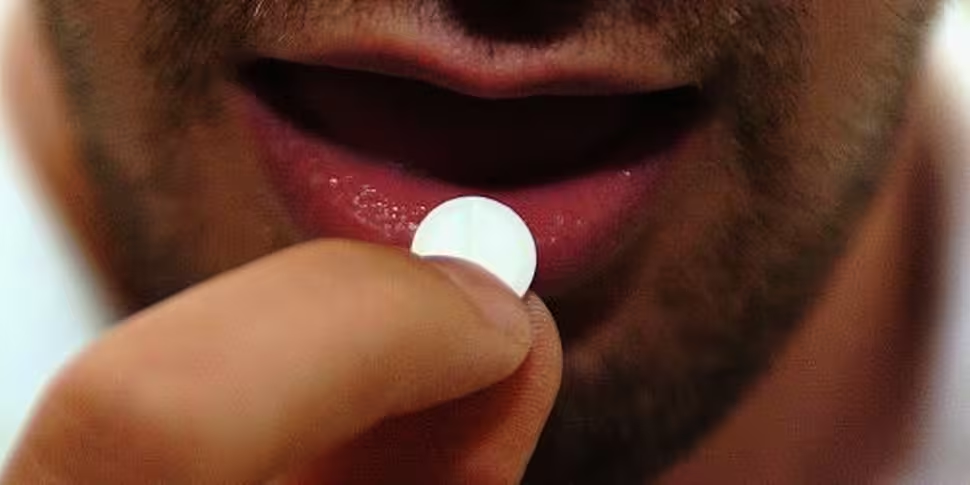
US regulators have approved the use of a 'digital pill' that tracks if a patient has taken their medication.
Abilify MyCite pills contain an ingestible sensor, that can record that the pill has been taken.
The US Food and Drug Administration (FDA) has approved the 'digital' version of the existing Abilify drug, which can be used to treat schizophrenia & bipolar disorder and as an "add-on treatment" for depression.
The sensor in the tablet communicates with a wearable patch, and the patch then transmits the information to a mobile app.
A patient can then keep track of the information on their smartphone, while they can also allow their caregivers and doctor access to the data.
BBC reports that the sensor - which is roughly the size of a grain of sand - starts communicating when it comes into contact with a patient's stomach fluid.
It is hoped that the technology will help some patients comply with their medication plan.
Mitchell Mathis, director of the Division of Psychiatry Products in the FDA’s Centre for Drug Evaluation and Research, explained: "Being able to track ingestion of medications prescribed for mental illness may be useful for some patients.
"The FDA supports the development and use of new technology in prescription drugs and is committed to working with companies to understand how technology might benefit patients and prescribers."
While experts have cautiously welcomed the potential of pills that monitor compliance, others raised concerns about the new technology.
In The New York Times, psychiatrist Dr Peter Kramer warned that "'digital drug' sounds like potentially coercive tool".